Tetracycline
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌtɛtrəˈsaɪkliːn/ |
| Trade names | Sumycin, others |
AHFS/Drugs.com | Monograph |
| MedlinePlus | a682098 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
Pharmacokinetic data | |
| Bioavailability | 75% |
| Metabolism | Not metabolized |
| Elimination half-life | 8–11 hours, 57–108 hours (kidney impairment) |
| Excretion | Urine (>60%), feces |
| Identifiers | |
| CAS Number |
|
PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
PDB ligand |
|
| ECHA InfoCard | 100.000.438 |
| Chemical and physical data | |
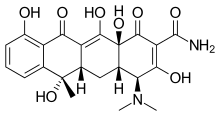
| Formula | C22H24N2O8 |
| Molar mass | 444.435 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
Tetracycline, sold under the brand name Sumycin among others, is an antibiotic used to treat a number of infections.[1] This includes acne, cholera, brucellosis, plague, malaria, and syphilis.[1] It is taken by mouth.[1]
Common side effects include vomiting, diarrhea, rash, and loss of appetite.[1] Other side effects include poor tooth development if used by children less than eight years of age, kidney problems, and sunburning easily.[1] Use during pregnancy may harm the baby.[1] Tetracycline is in the tetracyclines family of medications.[1] It works by blocking the ability of bacteria to make proteins.[1]
Tetracycline was patented in 1953 and came into commercial use in 1978.[2] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[3] Tetracycline is available as a generic medication.[1] The wholesale cost in the developing world is about 0.35 to 1.78 USD for a course of treatment.[4] In the United States a course of treatment typically costs less than 25 USD.[5] Tetracycline was originally made from bacteria of the Streptomyces type.[1]
Contents
1 Medical uses
1.1 Spectrum of bacterial susceptibility
1.2 Mechanisms of resistance
2 Precautions
2.1 Other uses
3 Mechanism of action
4 History
4.1 Evidence in antiquity
5 Society and culture
5.1 Price
5.2 Names
6 References
Medical uses
It is first-line therapy for Rocky Mountain spotted fever (Rickettsia), Lyme disease (B. burgdorferi), Q fever (Coxiella), psittacosis, and Mycoplasma pneumoniae and to eradicate nasal carriage of meningococci. Tetracycline tablets were used in the plague outbreak in India in 1994.[6]
Spectrum of bacterial susceptibility
Tetracyclines have a broad spectrum of antibiotic action. Originally, they possessed some level of bacteriostatic activity against almost all medically relevant aerobic and anaerobic bacterial genera, both Gram-positive and Gram-negative, with a few exceptions, such as Pseudomonas aeruginosa and Proteus spp., which display intrinsic resistance. However, acquired (as opposed to inherent) resistance has proliferated in many pathogenic organisms and greatly eroded the formerly vast versatility of this group of antibiotics. Resistance amongst Staphylococcus spp., Streptococcus spp., Neisseria gonorrhoeae, anaerobes, members of the Enterobacteriaceae, and several other previously sensitive organisms is now quite common. Tetracyclines remain especially useful in the management of infections by certain obligately intracellular bacterial pathogens such as Chlamydia, Mycoplasma, and Rickettsia. They are also of value in spirochaetal infections, such as syphilis, leptospirosis, and Lyme disease. Certain rare or exotic infections, including anthrax, plague and brucellosis, are also susceptible to tetracyclines. These agents also have activity against certain eukaryotic parasites, including those responsible for diseases such as malaria and balantidiasis. The following represents MIC susceptibility data for a few medically significant microorganisms:
Escherichia coli: 1 μg/mL — >128 μg/mL
Shigella spp.: 1 μg/mL — 128 μg/mL[7]
Mechanisms of resistance
Bacteria usually acquire resistance to tetracycline from horizontal transfer of a gene that either encodes an efflux pump or a ribosomal protection protein. Efflux pumps actively eject tetracycline from the cell, preventing the buildup of an inhibitory concentration of tetracycline in the cytoplasm.[8] Ribosomal protection proteins interact with the ribosome and dislodge tetracycline from the ribosome, allowing for translation to continue.[9]
Precautions
Use of the tetracycline antibiotics group is problematic; they can:[10]
- Discolor permanent teeth (yellow-gray-brown), from prenatal period through childhood and adulthood
- Be inactivated by Ca2+ions, so are not to be taken with milk, yogurt, and other dairy products
- Be inactivated by aluminium, iron, and zinc, not to be taken at the same time as indigestion remedies (common antacids and over-the-counter heartburn medicines)
- Cause skin photosensitivity, so exposure to the sun or intense light is not recommended
- Cause drug-induced lupus, and hepatitis
- Cause microvesicular fatty liver
- Cause tinnitus
- Interfere with methotrexate by displacing it from the various protein-binding sites
- Cause breathing complications, as well as anaphylactic shock, in some individuals
- Affect bone growth of the fetus, so should be avoided during pregnancy
Fanconi syndrome may result from ingesting expired tetracyclines.
Caution should be exercised in long-term use when breastfeeding. Short-term use is safe; bioavailability in milk is low to nil.[11] According to the U.S. Food and Drug Administration (FDA), cases of Stevens–Johnson syndrome, toxic epidermal necrolysis, and erythema multiforme associated with doxycyline use have been reported, but a causative role has not been established.[12]
Other uses

Tetracycline hydrochloride is available as yellow crystalline powder.
Since tetracycline is absorbed into bone, it is used as a marker of bone growth for biopsies in humans. Tetracycline labeling is used to determine the amount of bone growth within a certain period of time, usually a period around 21 days. Tetracycline is incorporated into mineralizing bone and can be detected by its fluorescence.[13] In "double tetracycline labeling", a second dose is given 11–14 days after the first dose, and the amount of bone formed during that interval can be calculated by measuring the distance between the two fluorescent labels.[14]
Tetracycline is also used as a biomarker in wildlife to detect consumption of medicine- or vaccine-containing baits.[15]
In genetic engineering, tetracycline is used in transcriptional activation.
It is also one of a group of antibiotics which together may be used to treat peptic ulcers caused by bacterial infections. The mechanism of action for the antibacterial effect of tetracyclines relies on disrupting protein translation in bacteria, thereby damaging the ability of microbes to grow and repair; however, protein translation is also disrupted in eukaryotic mitochondria leading to effects that may confound experimental results.[16][17]
Tetracycline has been used as an engineered "control switch" in chronic myelogenous leukemia models in mice. Engineers were able to develop a retrovirus that induced a particular type of leukemia in mice, and could then "switch" the cancer on and off through tetracycline administration. This could be used to grow the cancer in mice and then halt it at a particular stage to allow for further experimentation or study.[18]
A technique being developed for the control of the mosquito species Aedes aegypti uses a strain that is genetically modified to require tetracycline to develop beyond the larval stage. Modified males raised in a laboratory develop normally as they are supplied with this chemical and can be released into the wild. Their subsequent offspring inherit this trait, but find no tetracycline in their environments, so never develop into adults.[19]
Mechanism of action
Tetracycline inhibits protein synthesis by blocking the attachment of charged aminoacyl-tRNA to the A site on the ribosome. Tetracycline binds to the 30S subunit of microbial ribosomes. Thus, it prevents introduction of new amino acids to the nascent peptide chain.[20] The action is usually inhibitory and reversible upon withdrawal of the drug. Mammalian cells are less vulnerable to the effect of tetracyclines, despite the fact that tetracycline binds to the small ribosomal subunit of both prokaryotes and eukaryotes (30S and 40S, respectively). This is because bacteria actively pump tetracycline into their cytoplasm, even against a concentration gradient, whereas mammalian cells do not. This accounts for the relatively small off-site effect of tetracycline on human cells.[21]
History
The tetracyclines, a large family of antibiotics, were discovered as natural products by Benjamin Minge Duggar in 1945 and first prescribed in 1948.[22] Benjamin Duggar, working under Yellapragada Subbarow at Lederle Laboratories, discovered the first tetracycline antibiotic, chlortetracycline (Aureomycin), in 1945.[23]
In 1950, Harvard University professor R.B. Woodward determined the chemical structure of the related substance, oxytetracycline (Terramycin);[24][non-primary source needed] the patent protection for its fermentation and production was also first issued in that year.[citation needed] Chemist Lloyd Conover, in a research team of eight scientists at Pfizer, collaborated with Woodward over a two-year period, leading to tetracycline's discovery.[25][26][27]
Pfizer was of the view that it deserved the right to a patent on tetracycline and filed its Conover application in October 1952. Cyanamid filed its Boothe-Morton application for similar rights in March 1953, while Heyden Chemicals filed its Minieri application in September 1953, named after scientist P. Paul Minieri, to obtain a patent on tetracycline and its fermentation process.[28][full citation needed][non-primary source needed] This resulted in tetracycline litigation in which the winner would have to prove beyond reasonable doubt of priority invention and tetracycline's natural state.[clarification needed][needs update][29]
Evidence in antiquity
Nubian mummies studied in the 1990s were found to contain significant levels of tetracycline; the beer brewed at the time was conjectured to have been the source.[30]
Society and culture
Price
According to data from EvaluatePharma and published in the Boston Globe, the price of tetracycline rose from $0.06 per 250-mg pill in 2013 to $4.06 a pill in 2015.[31] The Globe described the "big price hikes of some generic drugs" as a "relatively new phenomenon" which has left most pharmacists "grappling" with large upswings" in the "costs of generics, with 'overnight' price changes sometimes exceeding 1,000%."[31]
Names
It is marketed under the brand names Sumycin, Tetracyn, and Panmycin, among others. Actisite is a thread-like fiber formulation used in dental applications.
It is also used to produce several semisynthetic derivatives, which together are known as the tetracycline antibiotics. The term "tetracycline" is also used to denote the four-ring system of this compound; "tetracyclines" are related substances that contain the same four-ring system.
References
^ abcdefghij "Tetracycline". The American Society of Health-System Pharmacists. Archived from the original on 28 December 2016. Retrieved 8 December 2016..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 489. ISBN 9783527607495. Archived from the original on 2016-12-20.
^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
^ "Tetracycline HCL". International Drug Price Indicator Guide. Archived from the original on 10 May 2017. Retrieved 8 December 2016.
^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 107. ISBN 9781284057560.
^ Lippincott's Illustrated Reviews: Pharmacology, 4th ed. Harvery RA, Champe, PC. Lippincott, Williams & Wilkins, 2009
^ http://www.toku-e.com/Assets/MIC/Tetracycline%20hydrochloride.pdf
^ Chopra I, Roberts M; Roberts (June 2001). "Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance". Microbiol. Mol. Biol. Rev. 65 (2): 232–260. doi:10.1128/MMBR.65.2.232-260.2001. PMC 99026. PMID 11381101.
^ Connell SR, Tracz DM, Nierhaus KH, Taylor DE (December 2003). "Ribosomal Protection Proteins and Their Mechanism of Tetracycline Resistance". Antimicrob. Agents Chemother. 47 (12): 3675–3681. doi:10.1128/AAC.47.12.3675-3681.2003. PMC 296194. PMID 14638464.
^ "Tetracycline: MedlinePlus Drug Information". medlineplus.gov. Archived from the original on 2017-05-10. Retrieved 2017-05-19.
^ Riordan, Jan."Breastfeeding & Human Lactation", Jones & Bartlett,2010 p.179
^ FDA Adverse Events Reporting System Archived 2011-01-17 at the Wayback Machine. Retrieved on January 14, 2011
^ Mayton CA. Tetracycline labeling of bone Archived 2007-03-12 at the Wayback Machine.
^ The Johns Hopkins Medical Institutions. > Tetracycline Labeling Archived 2012-12-15 at Archive.is Last updated January 8, 2001.
^ Olson CA, Mitchell KD, Werner PA (October 2000). "Bait ingestion by free-ranging raccoons and nontarget species in an oral rabies vaccine field trial in Florida". J. Wildl. Dis. 36 (4): 734–43. doi:10.7589/0090-3558-36.4.734. PMID 11085436. Archived from the original on 2013-04-15.
^ Moullan N, Mouchiroud L, Wang X, Ryu D, Williams EG, Mottis A, Jovaisaite V, Frochaux MV, Quiros PM, Deplancke B, Houtkooper RH, Auwerx J (2015). "Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research". Cell Reports. 10 (10): 1681–91. doi:10.1016/j.celrep.2015.02.034. PMID 25772356.
^ Chatzispyrou IA, Held NM, Mouchiroud L, Auwerx J, Houtkooper RH (2015). "Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research". Cancer Research. 75 (21): 4446–9. doi:10.1158/0008-5472.CAN-15-1626. PMID 26475870.
^ A Dugray, JF Geay, A Foudi, ML Bonnet, W Vainchenker, F Wendling, F Louache & AG Turhan. "Rapid generation of a tetracycline-inducible BCR-ABL defective retrovirus using a single autoregulatory retroviral cassette".CS1 maint: Multiple names: authors list (link)
^ Conal Urquhart (15 July 2012). "Can GM mosquitoes rid the world of a major killer?". The Observer. Archived from the original on 5 December 2013. Retrieved 2012-07-15.
^ Mehta, Akul (2011-05-27). "Mechanism of Action of Tetracyclines". Pharmaxchange.info. Archived from the original on 2012-06-05. Retrieved 2012-06-07.
^ Kenneth Todar, Antimicrobial Agents in the Treatment of Infectious Disease. Online Textbook of Bacteriology. 2012. "Archived copy". Archived from the original on 2013-10-08. Retrieved 2013-08-27.CS1 maint: Archived copy as title (link)
^ Klajn, Rafal, Chemistry and chemical biology of tetracyclines Archived 2007-06-17 at the Wayback Machine., retrieved 20 June 2007.[better source needed]
^ Jukes, Thomas H. (1985). "Some Historical Notes on Chlortetracycline". Reviews of Infectious Diseases. Washington, DC. 7 (5, Sep.-Oct.): 702–707. JSTOR 4453725.
^ Hochstein, F. A.; Stephens, C. R.; Conover, L. H.; Regna, P. P.; Pasternack, R.; Gordon, P. N.; Pilgrim, F. J.; Brunings, K. J.; Woodward, R. B. (November 1953). "The structure of terramycin". Journal of the American Chemical Society. 75 (22): 5455–75. doi:10.1021/ja01118a001.
[non-primary source needed]
^ The team included K.J. Brunings, Francis A. Hochstein, Frederick J. Pilgrim, C.R. Stephens, Lloyd Conover, Abraham Bavley, Richard Pasternack, and Peter P. Regna.[citation needed]
^ TSNL Staff (August 9, 1952). "Coronagraph Mounts Done". The Science News-Letter. Washington, DC. 62 (6): 83. doi:10.2307/3931295. JSTOR 3931295.
^ WSJ Staff (July 28, 1952). "Scientists Discover Terramycin's Secret: Its Complex Structure". The Wall Street Journal. Archived from the original on March 14, 2017. Retrieved March 13, 2017. (Subscription required (help)).[Quote:] Laboratory Work May Lead to Manufacture of Non-Toxic Antibiotic Drugs
^ Patented February 7, 1956[full citation needed][non-primary source needed]
^ Prior to 1952, neither the molecular structure of Terramycin nor that of Aureomycin was known.[citation needed] In the spring of 1952, the Pfizer team succeeded in ascertaining the structures of both Terramycin (see Hochstein et al., op. cit.) and Aureomycin.[citation needed] Shortly thereafter, Lloyd Conover produced another antibiotic, tetracycline, that he discovered to be the result of dechlorination of Aureomycin.[citation needed] Pfizer filed the application for a product and process patent on tetracycline in October 1952.[citation needed] In March 1953, Cyanamid filed its Boothe-Morton application for a similar patent.[citation needed] In September 1953, Heyden Chemicals filed for a patent on tetracycline and the fermentation process for producing it in the name of P. Paul Minieri (see the Minieri patent cited in the main body, op. cit.). In October 1953, Bristol filed a similar application under the name of "Heinemann".[citation needed] Because of an agreement among the major drug companies to cross-license tetracyline, the Federal Trade Commission (FTC) initiated Fair Trade Practices litigation that remained unresolved until 1982.[citation needed] The FTC argued that tetracycline was not patentable because of its production through fermentation,[citation needed] that Pfizer, American Cyanamid (successor to Heyden), Bristol-Myers and others had conspired to fix prices for the new antibiotic,[citation needed] and that distribution of such fermented, nonsynthetic products, because not patentable, was subject to the FTC price-fixing challenge.[citation needed]
^ Armelagos, George (2000). "Take Two Beers and Call Me in 1,600 Years: Use of Tetracycline by Nubians and Ancient Egyptians" (PDF). Natural History (5, May): 50–53. Retrieved March 13, 2017.
^ ab McCluskey, Priyanka Dayal (6 November 2015). "As competition wanes, prices for generics skyrocket". Boston Globe. Archived from the original on 19 November 2015. Retrieved 18 November 2015.

Comments
Post a Comment