Formic acid
| |||
 | |||
| Names | |||
|---|---|---|---|
Preferred IUPAC name Formic acid[1] | |||
Systematic IUPAC name Methanoic acid[1] | |||
| Other names Carbonous acid; Formylic acid; Hydrogen carboxylic acid; Hydroxy(oxo)methane; Metacarbonoic acid; Oxocarbinic acid; Oxomethanol | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
ChEBI |
| ||
ChEMBL |
| ||
ChemSpider |
| ||
DrugBank |
| ||
ECHA InfoCard | 100.000.527 | ||
EC Number | 200-579-1 | ||
E number | E236 (preservatives) | ||
KEGG |
| ||
PubChem CID |
| ||
RTECS number | LQ4900000 | ||
UNII |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | CH2O2 | ||
Molar mass | 46.03 g·mol−1 | ||
| Appearance | Colorless fuming liquid | ||
Odor | Pungent, penetrating | ||
Density | 1.220 g/mL | ||
Melting point | 8.4 °C (47.1 °F; 281.5 K) | ||
Boiling point | 100.8 °C (213.4 °F; 373.9 K) | ||
Solubility in water | Miscible | ||
Solubility | Miscible with ether, acetone, ethyl acetate, glycerol, methanol, ethanol Partially soluble in benzene, toluene, xylenes | ||
log P | −0.54 | ||
Vapor pressure | 35 mmHg (20 °C)[2] | ||
Acidity (pKa) | 3.77[3] | ||
Magnetic susceptibility (χ) | -19.90·10−6 cm3/mol | ||
Refractive index (nD) | 1.3714 (20 °C) | ||
Viscosity | 1.57 cP at 268 °C | ||
| Structure | |||
Molecular shape | Planar | ||
Dipole moment | 1.41 D (gas) | ||
| Thermochemistry | |||
Std molar entropy (S | 131.8 J/mol K | ||
Std enthalpy of formation (ΔfH | −425.0 kJ/mol | ||
Std enthalpy of combustion (ΔcH | −254.6 kJ/mol | ||
| Pharmacology | |||
ATCvet code | QP53AG01 (WHO) | ||
| Hazards | |||
| Main hazards | Corrosive; irritant; sensitizer | ||
Safety data sheet | See: data page JT Baker | ||
R-phrases (outdated) | R10 R35 | ||
S-phrases (outdated) | (S1/2) S23 S26 S45 | ||
NFPA 704 |  2 3 1 | ||
Flash point | 69 °C (156 °F; 342 K) | ||
Autoignition temperature | 601 °C (1,114 °F; 874 K) | ||
Explosive limits | 14–34%[citation needed] 18%–57% (90% solution)[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 700 mg/kg (mouse, oral), 1100 mg/kg (rat, oral), 4000 mg/kg (dog, oral)[4] | ||
LC50 (median concentration) | 7853 ppm (rat, 15 min) 3246 ppm (mouse, 15 min)[4] | ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) | TWA 5 ppm (9 mg/m3)[2] | ||
REL (Recommended) | TWA 5 ppm (9 mg/m3)[2] | ||
IDLH (Immediate danger) | 30 ppm[2] | ||
| Related compounds | |||
Related carboxylic acids | Acetic acid Propionic acid | ||
Related compounds | Formaldehyde Methanol | ||
Supplementary data page | |||
Structure and properties | Refractive index (n), Dielectric constant (εr), etc. | ||
Thermodynamic data | Phase behaviour solid–liquid–gas | ||
Spectral data | UV, IR, NMR, MS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Infobox references | |||
Formic acid, systematically named methanoic acid, is the simplest carboxylic acid. The chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. The word "formic" comes from the Latin word for ant, formica, referring to its early isolation by the distillation of ant bodies. Esters, salts, and the anion derived from formic acid are called formates. Industrially formic acid is produced from methanol.
Contents
1 Properties
2 Natural occurrence
3 Production
3.1 From methyl formate and formamide
3.2 Niche chemical routes
3.2.1 By-product of acetic acid production
3.2.2 Hydrogenation of carbon dioxide
3.2.3 Oxidation of biomass
3.2.4 Laboratory methods
3.3 Biosynthesis
4 Uses
5 Chemical reactions
6 Reactions
6.1 Decomposition
6.2 Addition to alkenes
6.3 Formic acid anhydride
7 History
8 Safety
9 See also
10 References
11 External links
Properties

Cyclic dimer of formic acid; dashed green lines represent hydrogen bonds
Formic acid is a colorless liquid having a pungent, penetrating odor[5] at room temperature, not unlike the related acetic acid. It is miscible with water and most polar organic solvents, and is somewhat soluble in hydrocarbons. In hydrocarbons and in the vapor phase, it consists of hydrogen-bonded dimers rather than individual molecules.[6][7] Owing to its tendency to hydrogen-bond, gaseous formic acid does not obey the ideal gas law.[7] Solid formic acid (two polymorphs) consists of an effectively endless network of hydrogen-bonded formic acid molecules. This relatively complicated compound also forms a low-boiling azeotrope with water (22.4%) and liquid formic acid also tends to supercool.
Natural occurrence
In nature, formic acid is found in most ants and in stingless bees of the Oxytrigona genus.[8][9] The wood ants from the genus Formica can spray formic acid on their prey or to defend the nest. It is also found in the trichomes of stinging nettle (Urtica dioica). Formic acid is a naturally occurring component of the atmosphere due primarily to forest emissions.[10]
Production
In 2009, the worldwide capacity for producing formic acid was 720,000 tonnes/annum, roughly equally divided between Europe (350,000, mainly in Germany) and Asia (370,000, mainly in China) while production was below 1000 tonnes/annum in all other continents.[11] It is commercially available in solutions of various concentrations between 85 and 99 w/w %.[6] As of 2009[update], the largest producers are BASF, Eastman Chemical Company, LC Industrial, and Feicheng Acid Chemicals, with the largest production facilities in Ludwigshafen (200,000 tonnes/annum, BASF, Germany), Oulu (105,000, Eastman, Finland), Nakhon Pathom (n/a, LC Industrial) and Feicheng (100,000, Feicheng, China). 2010 prices ranged from around €650/tonne (equivalent to around $800/tonne) in Western Europe to $1250/tonne in the United States.[11]
From methyl formate and formamide
When methanol and carbon monoxide are combined in the presence of a strong base, the result is methyl formate, according to the chemical equation:[6]
- CH3OH + CO → HCO2CH3
In industry, this reaction is performed in the liquid phase at elevated pressure. Typical reaction conditions are 80 °C and 40 atm. The most widely used base is sodium methoxide. Hydrolysis of the methyl formate produces formic acid:
- HCO2CH3 + H2O → HCO2H + CH3OH
Efficient hydrolysis of methyl formate requires a large excess of water. Some routes proceed indirectly by first treating the methyl formate with ammonia to give formamide, which is then hydrolyzed with sulfuric acid:
- HCO2CH3 + NH3 → HC(O)NH2 + CH3OH
- 2 HC(O)NH2 + 2H2O + H2SO4 → 2HCO2H + (NH4)2SO4
A disadvantage of this approach is the need to dispose of the ammonium sulfate byproduct. This problem has led some manufacturers to develop energy-efficient methods of separating formic acid from the excess water used in direct hydrolysis. In one of these processes (used by BASF) the formic acid is removed from the water by liquid-liquid extraction with an organic base.[citation needed]
Niche chemical routes
By-product of acetic acid production
A significant amount of formic acid is produced as a byproduct in the manufacture of other chemicals. At one time, acetic acid was produced on a large scale by oxidation of alkanes, by a process that cogenerates significant formic acid.[citation needed] This oxidative route to acetic acid is declining in importance, so that the aforementioned dedicated routes to formic acid have become more important.
Hydrogenation of carbon dioxide
The catalytic hydrogenation of CO2 to formic acid has long been studied. This reaction can be conducted homogeneously.[12][13]
Oxidation of biomass
Formic acid can also be obtained by aqueous catalytic partial oxidation of wet biomass (OxFA process).[14][15] A Keggin-type polyoxometalate (H5PV2Mo10O40) is used as the homogeneous catalyst to convert sugars, wood, waste paper or cyanobacteria to formic acid and CO2 as the sole byproduct. Yields of up to 53% formic acid can be achieved.[citation needed]
Laboratory methods
In the laboratory, formic acid can be obtained by heating oxalic acid in glycerol and extraction by steam distillation.[16] Glycerol acts as a catalyst, as the reaction proceeds through a glyceryl oxalate intermediary. If the reaction mixture is heated to higher temperatures, allyl alcohol results. The net reaction is thus:
- C2O4H2 → CO2H2 + CO2
Another illustrative method involves the reaction between lead formate and hydrogen sulfide, driven by the formation of lead sulfide.[17]
- Pb(HCOO)2 + H2S → 2HCOOH + PbS
Biosynthesis
Formic acid is named after ants which have high concentrations of the compound in their venom. In ants formic acid is derived from serine through a 5,10-Methenyltetrahydrofolate intermediate.[18] The conjugate base of formic acid, formate, also occurs widely in nature. An assay for formic acid in body fluids, designed for determination of formate after methanol poisoning, is based on the reaction of formate with bacterial formate dehydrogenase.[19]
Uses
A major use of formic acid is as a preservative and antibacterial agent in livestock feed. In Europe, it is applied on silage (including fresh hay) to promote the fermentation of lactic acid and to suppress the formation of butyric acid; it also allows fermentation to occur quickly, and at a lower temperature, reducing the loss of nutritional value.[6] Formic acid arrests certain decay processes and causes the feed to retain its nutritive value longer, and so it is widely used to preserve winter feed for cattle.[20] In the poultry industry, it is sometimes added to feed to kill E. coli bacteria.[21][22] Use as preservative for silage and (other) animal feed constituted 30% of the global consumption in 2009.[11]
Formic acid is also significantly used in the production of leather, including tanning (23% of the global consumption in 2009[11]), and in dyeing and finishing textiles (9% of the global consumption in 2009[11]) because of its acidic nature. Use as a coagulant in the production of rubber[6] consumed 6% of the global production in 2009.[11]
Formic acid is also used in place of mineral acids for various cleaning products,[6] such as limescale remover and toilet bowl cleaner. Some formate esters are artificial flavorings or perfumes.
Beekeepers use formic acid as a miticide against the tracheal mite (Acarapis woodi) and the Varroa destructor mite and Varroa jacobsoni mite.[23]
Formic acid application has been reported to be an effective treatment for warts.[24]
Formic acid is being investigated for use in fuel cells.[25][26]
Formic acid is often used as a component of mobile phase in reversed-phase high-performance liquid chromotagraphy (RP-HPLC) analysis and separation techniques for the separation of hydrophobic macromolecules, such as peptides, proteins and more complex structures including intact viruses. Especially when paired with mass spectrometry detection, formic acid offers several advantages over the more traditionally used phosphoric acid.[27][28]
Chemical reactions
Formic acid is about ten times stronger than acetic acid. It is used as a volatile pH modifier in HPLC and capillary electrophoresis.
Formic acid is a source for a formyl group for example in the formylation of methylaniline to N-methylformanilide in toluene.[29]
In synthetic organic chemistry, formic acid is often used as a source of hydride ion. The Eschweiler-Clarke reaction and the Leuckart-Wallach reaction are examples of this application. It, or more commonly its azeotrope with triethylamine, is also used as a source of hydrogen in transfer hydrogenation.
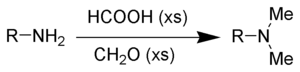
As mentioned below, formic acid readily decomposes with concentrated sulfuric acid to form carbon monoxide.
- CH2O2 + H2SO4 → H2SO4< + H2O + CO
Reactions
Formic acid shares most of the chemical properties of other carboxylic acids. Because of its high acidity, solutions in alcohols form esters spontaneously. Formic acid shares some of the reducing properties of aldehydes, reducing solutions of gold, silver, and platinum to the metals.[citation needed]
Decomposition
Heat and especially acids cause formic acid to decompose to carbon monoxide (CO) and water (dehydration). Treatment of formic acid with sulfuric acid is a convenient laboratory source of CO.[30][31]
In the presence of platinum, it decomposes with a release of hydrogen and carbon dioxide.
- CH2O2 → H2 + CO2
Soluble ruthenium catalysts are also effective.[32][33] Carbon monoxide free hydrogen has been generated in a very wide pressure range (1–600 bar).[32] Formic acid has been considered as a means of hydrogen storage.[34] The co-product of this decomposition, carbon dioxide, can be rehydrogenated back to formic acid in a second step. Formic acid contains 53 g L−1 hydrogen at room temperature and atmospheric pressure, which is three and a half times as much as compressed hydrogen gas can attain at 350 bar pressure (14.7 g L−1). Pure formic acid is a liquid with a flash point of +69 °C, much higher than that of gasoline (−40 °C) or ethanol (+13 °C).[citation needed]
Addition to alkenes
Formic acid is unique among the carboxylic acids in its ability to participate in addition reactions with alkenes. Formic acids and alkenes readily react to form formate esters. In the presence of certain acids, including sulfuric and hydrofluoric acids, however, a variant of the Koch reaction occurs instead, and formic acid adds to the alkene to produce a larger carboxylic acid.[35]
Formic acid anhydride
An unstable formic anhydride, H(C=O)−O−(C=O)H, can be obtained by dehydration of formic acid with N,N'-dicyclohexylcarbodiimide in ether at low temperature.[36]
History
Some alchemists and naturalists were aware that ant hills give off an acidic vapor as early as the 15th century. The first person to describe the isolation of this substance (by the distillation of large numbers of ants) was the English naturalist John Ray, in 1671.[37][38] Ants secrete the formic acid for attack and defense purposes. Formic acid was first synthesized from hydrocyanic acid by the French chemist Joseph Gay-Lussac. In 1855, another French chemist, Marcellin Berthelot, developed a synthesis from carbon monoxide similar to the process used today.
Formic acid was long considered a chemical compound of only minor interest in the chemical industry. In the late 1960s, however, significant quantities became available as a byproduct of acetic acid production. It now finds increasing use as a preservative and antibacterial in livestock feed.
Safety
Formic acid has low toxicity (hence its use as a food additive), with an LD50 of 1.8 g/kg (tested orally on mice). The concentrated acid is corrosive to the skin.[6]
Formic acid is readily metabolized and eliminated by the body. Nonetheless, it has specific toxic effects; the formic acid and formaldehyde produced as metabolites of methanol are responsible for the optic nerve damage, causing blindness seen in methanol poisoning.[39] Some chronic effects of formic acid exposure have been documented. Some experiments on bacterial species have demonstrated it to be a mutagen.[40] Chronic exposure in humans may cause kidney damage.[40] Another possible effect of chronic exposure is development of a skin allergy that manifests upon re-exposure to the chemical.
Concentrated formic acid slowly decomposes to carbon monoxide and water, leading to pressure buildup in the containing vessel. For this reason, 98% formic acid is shipped in plastic bottles with self-venting caps.
The hazards of solutions of formic acid depend on the concentration. The following table lists the EU classification of formic acid solutions:
Concentration (weight percent) | Classification | R-Phrases |
|---|---|---|
| 2%–10% | Irritant (Xi) | R36/38 |
| 10%–90% | Corrosive (C) | R34 |
| >90% | Corrosive (C) | R35 |
Formic acid in 85% concentration is flammable, and diluted formic acid is on the U.S. Food and Drug Administration list of food additives.[41] The principal danger from formic acid is from skin or eye contact with the concentrated liquid or vapors. The U.S. OSHA Permissible Exposure Level (PEL) of formic acid vapor in the work environment is 5 parts per million parts of air (ppm).
See also
- Orthoformic acid
References
^ ab Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 745. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abcde "NIOSH Pocket Guide to Chemical Hazards #0296". National Institute for Occupational Safety and Health (NIOSH).
^ Brown, H. C. et al., in Braude, E. A. and Nachod, F. C., Determination of Organic Structures by Physical Methods, Academic Press, New York, 1955.
^ ab "Formic acid". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health. 4 December 2014. Retrieved 26 March 2015.
^ "OSHA Occupational Chemical Database - Occupational Safety and Health Administration". www.osha.gov.
^ abcdefg Reutemann, Werner; Kieczka, Heinz (2000). "Formic Acid". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a12_013. ISBN 978-3-527-30673-2.
^ ab Balabin R. M. (2009). "Polar (Acyclic) Isomer of Formic Acid Dimer: Gas-Phase Raman Spectroscopy Study and Thermodynamic Parameters". The Journal of Physical Chemistry A. 113 (17): 4910–8. Bibcode:2009JPCA..113.4910B. doi:10.1021/jp9002643. PMID 19344174.
^ Hoffman, Donald R (2010). "Ant venoms". Current Opinion in Allergy and Clinical Immunology. 10 (4): 342–6. doi:10.1097/ACI.0b013e328339f325. PMID 20445444.
^ Roubik, DW; Smith, BH; Carlson, RG (1987). "Formic acid in caustic cephalic secretions of stingless bee,Oxytrigona (Hymenoptera: Apidae)". J Chem Ecol. 13 (5): 1079–86. doi:10.1007/BF01020539. PMID 24302133.
^ Sanhueza, Eugenio; Andreae, Meinrat O (1991). "Emission of formic and acetic acids from tropical Savanna soils". Geophysical Research Letters. 18 (9): 1707–10. Bibcode:1991GeoRL..18.1707S. doi:10.1029/91GL01565.
^ abcdef S. N. Bizzari; M. Blagoev (June 2010). "CEH Marketing Research Report: FORMIC ACID". Chemical Economics Handbook. SRI consulting. Retrieved July 2011. Check date values in:|accessdate=(help)
^ P. G. Jessop, in Handbook of Homogeneous Hydrogenation (Eds.: J. G. de Vries, C. J. Elsevier), Wiley-VCH, Weinheim, Germany, 2007, pp. 489–511.
^ Jessop, Philip G; Joó, Ferenc; Tai, Chih-Cheng (2004). "Recent advances in the homogeneous hydrogenation of carbon dioxide". Coordination Chemistry Reviews. 248 (21–24): 2425. doi:10.1016/j.ccr.2004.05.019.
^ Wölfel, Rene; Taccardi, Nicola; Bösmann, Andreas; Wasserscheid, Peter (2011). "Selective catalytic conversion of biobased carbohydrates to formic acid using molecular oxygen". Green Chemistry. 13 (10): 2759. doi:10.1039/C1GC15434F.
^ Albert, Jakob; Wölfel, Rene; Bösmann, Andreas; Wasserscheid, Peter (2012). "Selective oxidation of complex, water-insoluble biomass to formic acid using additives as reaction accelerators". Energy & Environmental Science. 5 (7): 7956. doi:10.1039/C2EE21428H.
^ Chattaway, Frederick Daniel (1914). "XX.—Interaction of glycerol and oxalic acid". Journal of the Chemical Society, Transactions. 105: 151–6. doi:10.1039/CT9140500151. hdl:2027/mdp.39015067135775.
^ Arthur Sutcliffe (1930) Practical Chemistry for Advanced Students (1949 Ed.), John Murray, London.
^ Hefetz, Abraham; Blum, Murray (1 Nov 1978). "Biosynthesis of formic acid by the poison glands of formicine ants". Biochimica et Biophysica Acta (BBA) - General Subjects. 543 (4): 484–496. doi:10.1016/0304-4165(78)90303-3. PMID 718985.
^ Makar, A.B; McMartin, K.E; Palese, M; Tephly, T.R (1975). "Formate assay in body fluids: Application in methanol poisoning". Biochemical Medicine. 13 (2): 117–26. doi:10.1016/0006-2944(75)90147-7. PMID 1.
^ Organic Acids and Food Preservation, Maria M. Theron, J. F. Rykers Lues
^ Griggs, J. P; Jacob, J. P (2005). "Alternatives to Antibiotics for Organic Poultry Production". The Journal of Applied Poultry Research. 14 (4): 750. doi:10.1093/japr/14.4.750.
^ Garcia, V; Catala-Gregori, P; Hernandez, F; Megias, M. D; Madrid, J (2007). "Effect of Formic Acid and Plant Extracts on Growth, Nutrient Digestibility, Intestine Mucosa Morphology, and Meat Yield of Broilers". The Journal of Applied Poultry Research. 16 (4): 555. doi:10.3382/japr.2006-00116.
^ Hoppe, H., Ritter, W., & Stephen, E. W. C. (1989). "The control of parasitic bee mites: Varroa jacobsoni, Acarapis woodi and Tropilaelaps clareae with formic acid". American Bee Journal.CS1 maint: Multiple names: authors list (link)
^ Bhat, Ramesh M; Vidya, Krishna; Kamath, Ganesh (2001). "Topical formic acid puncture technique for the treatment of common warts". International Journal of Dermatology. 40 (6): 415–9. doi:10.1046/j.1365-4362.2001.01242.x. PMID 11589750.
^ Ha, S; Larsen, R; Masel, R.I (2005). "Performance characterization of Pd/C nanocatalyst for direct formic acid fuel cells". Journal of Power Sources. 144 (1): 28–34. Bibcode:2005JPS...144...28H. doi:10.1016/j.jpowsour.2004.12.031.
^ Jorn Madslien (27 June 2017). "Ant power: Take a ride on a bus that runs on formic acid". BBC News. Retrieved 11 July 2017.
^ https://www.novapublishers.com/catalog/product_info.php?products_id=48192[full citation needed]
^ Heukeshoven, Jochen; Dernick, Rudolf (1982). "Reversed-phase high-performance liquid chromatography of virus proteins and other large hydrophobic proteins in formic acid containing solvents". Journal of Chromatography A. 252: 241–54. doi:10.1016/S0021-9673(01)88415-6. PMID 6304128.
^ L. F. Fieser; J. E. Jones (1955). "N-Methylformanilide". Organic Syntheses.; Collective Volume, 3, p. 590
^ Koch, H.; Haaf, W. (1973). "1-Adamantanecarboxylic Acid". Organic Syntheses.CS1 maint: Multiple names: authors list (link) ; Collective Volume, 5, p. 20
^ G. H. Coleman, David Craig (1943). "p-Tolualdehyde". Organic Syntheses.; Collective Volume, 2, p. 583
^ ab Fellay, Céline; Dyson, Paul J.; Laurenczy, Gábor (2008). "A Viable Hydrogen-Storage System Based on Selective Formic Acid Decomposition with a Ruthenium Catalyst". Angewandte Chemie International Edition. 47 (21): 3966–8. doi:10.1002/anie.200800320. PMID 18393267.
^ G. Laurenczy, C. Fellay, P. J. Dyson, Hydrogen production from formic acid. PCT Int. Appl. (2008), 36pp. CODEN: PIXXD2 WO 2008047312 A1 20080424 AN 2008:502691
^ Joó, Ferenc (2008). "Breakthroughs in Hydrogen Storage-Formic Acid as a Sustainable Storage Material for Hydrogen". Chem Sus Chem. 1 (10): 805–8. doi:10.1002/cssc.200800133. PMID 18781551.
^ Haaf, Wolfgang (1966). "Die Synthese sekundärer Carbonsäuren nach der Ameisensäure-Methode". Chemische Berichte. 99 (4): 1149–52. doi:10.1002/cber.19660990410.
^ Wu, G; Shlykov, S; Van Alseny, F. S; Geise, H. J; Sluyts, E; Van Der Veken, B. J (1995). "Formic Anhydride in the Gas Phase, Studied by Electron Diffraction and Microwave and Infrared Spectroscopy, Supplemented with Ab-Initio Calculations of Geometries and Force Fields". The Journal of Physical Chemistry. 99 (21): 8589–98. doi:10.1021/j100021a022.
^ Wray, J (1670). "Extract of a Letter, Written by Mr. John Wray to the Publisher January 13. 1670. Concerning Some Un-Common Observations and Experiments Made with an Acid Juyce to be Found in Ants". Philosophical Transactions of the Royal Society of London. 5 (57–68): 2063–2066. doi:10.1098/rstl.1670.0052.
^ Johnson, W. B. (1803). History of the process and present state of animal chemistry.
^ Sadun, A. A (2002). "Mitochondrial optic neuropathies". Journal of Neurology, Neurosurgery, and Psychiatry. 72 (4): 423–5. doi:10.1136/jnnp.72.4.423. PMC 1737836. PMID 11909893.
^ ab "Occupational Safety and Health Guideline for Formic Acid". OSHA. Retrieved 28 May 2011.
^ U.S. Code of Federal Regulations: 21 CFR 186.1316, 21 CFR 172.515
External links
| Wikimedia Commons has media related to Formic acid. |
Wikisource has the text of the 1911 Encyclopædia Britannica article Formic Acid. |
Carbon monoxide as reagent in the formylation of aromatic compounds.
International Chemical Safety Card 0485.
NIOSH Pocket Guide to Chemical Hazards.
ChemSub Online (Formic acid).
Formic Acid Use in Beekeeping: Handbook and Manual of Treatments.
Formic Acid Use by ants: topical application on the cuticle.




Comments
Post a Comment