Heterogeneous catalysis
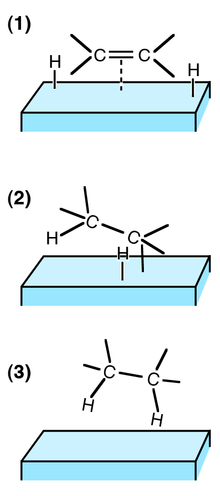
Hydrogenation of ethene on a solid surface
In chemistry, heterogeneous catalysis also refers to the form of catalysis where the phase of the catalyst differs from that of the reactants. Phase here refers not only to solid, liquid, vs gas, but also immiscible liquids, e.g. oil and water. The great majority of practical heterogeneous catalysts are solids and the great majority of reactants are gases or liquids.[1] Heterogeneous catalysis is of paramount importance in many areas of the chemical and energy industries. Heterogeneous catalysis has attracted Nobel prizes for Fritz Haber in 1918, Carl Bosch in 1931, Irving Langmuir in 1932, and Gerhard Ertl in 2007.[2][3][4][5][6]
Contents
1 Adsorption
1.1 Types of adsorption
2 Surface reactions
3 Catalyst deactivation
4 Classes of heterogeneous catalysts
5 Examples
5.1 Other examples
6 See also
7 References
Adsorption
Adsorption is commonly an essential first step in heterogeneous catalysis. Adsorption is when a molecule in the gas phase or in solution binds to atoms on the solid or liquid surface. The molecule that is binding is called the adsorbate, and the surface to which it binds is the adsorbent. The process of the adsorbate binding to the adsorbent is called adsorption. The reverse of this process (the adsorbate splitting from adsorbent) is called desorption. In terms of catalyst support, the catalyst is the adsorbate and the support is the adsorbent.
Types of adsorption
Two types of adsorption are recognized in heterogeneous catalysis, although many processes fall into an ambiguous range between the two extremes. In the first type, physisorption, induces only small changes to the electronic structure of the adsorbate. Typical energies for physisorption are from 2 to 10 kcal/mol. The second type is chemisorption, in which the adsorbate is strongly perturbed, often with bond-breaking. Energies for typical chemisorptions range from 15 to 100 kcal/mol.
For physisorption, adsorbate is attracted to the surface atoms by van der Waals forces. A mathematical model for the physisorption was developed by London to predict the energies of basic physisorption of non-polar molecules. The analysis of physisorption for polar or ionic species is more complex.
Chemisorption results in the sharing of electrons between the adsorbate and the adsorbent. Chemisorption is traditionally described by the Lennard-Jones potential, which considers various cases, two of which are.
- Molecular adsorption, where the adsorbate remains intact. An example is alkene binding by platinum.
- In dissociation adsorption, one or more bonds break concomitantly with adsorption. In this case the barrier to dissociation affects the rate of adsorption. An example of this the binding of H2, where the H-H bond is broken upon adsorption [7] by hydrogen spillover.
Surface reactions
In heterogeneous catalysis, the reactants diffuse to the catalyst surface and adsorb onto it, via the formation of chemical bonds. After reaction, the products desorb from the surface and diffuse away. Understanding the transport phenomena and surface chemistry such as dispersion is important. If diffusion rates are not taken into account, the reaction rates for various reactions on surfaces depend solely on the rate constants and reactant concentrations.
For solid heterogeneous catalysts, the surface area of the catalyst is critical since it determines the availability of catalytic sites. Surface areas can be large, for example some mesoporous silicates have areas of 1000 m2/g. The most common approach to maximizing surface area is by the use of catalyst supports, which are the materials over which the catalysts are spread.
With supported catalysts, reaction canoccurs often occurs on the surface of either the catalyst or the support. In terms of surface reactions three mechanisms apply.
- Langmuir-Hinshelwood mechanism. The two molecules A and B both adsorb to the surface. While adsorbed to the surface, the A and B "meet," bond, and then the new molecule A-B desorbs.
- Rideal-Eley mechanism. One of the two molecules, A, adsorbs to the surface. The second molecule, B, meets A on the surface, having never adsorbed to the surface, and they react and bind. Then the newly formed A-B desorbs.
- Precursor mechanism. One of the two molecules, A, is adsorbed on the surface. The second molecule, B, collides with the surface, forming a mobile precursor state. The molecule B then collides with A on the surface, they react, bind and the new molecule desorbs.
Any surface reaction can be described as following one of these mechanisms, or some combination of these mechanisms. In addition, all of these above mechanisms can occur in reverse. In general, the pathway for a reaction on a surface is as follows. First the reactants adsorb onto the surface. Through a series of bonds being formed and being broken, adsorbed intermediates are produced and destroyed. Then the final product(s) is produced and it desorbs from the solid. Most metal surface reaction occur by chain propagation.[7]
Catalyst deactivation
Heterogeneous catalyst lose their activity (deactivate) by many pathways. Mechanisms include coking, sintering, and poisoning. In some cases, the catalyst undergoes solid-state transformation.[8][9]
Classes of heterogeneous catalysts
Although the majority of heterogeneous catalysts are solids, many variations exist.
| Reacting phases |
Examples given |
Comment |
|---|---|---|
| solid + gas |
Ammonia synthesis from N2 + H2 over iron catalysts |
|
| solid + solution |
hydrogenation of fatty acids with nickel |
used for the production of margarine |
| immiscible liquid phases |
hydroformylation of propene |
catalyst in aqueous phase, reactants and products mainly in nonaqueous phase |
Examples
Many examples exist, the table emphasizes large-scale industrial processes,[10] although diverse examples are known.
| Process |
Reactants, product(s) |
Catalyst |
Comment |
|---|---|---|---|
| Sulfuric acid synthesis (Contact process) |
SO2 + O2, SO3 |
vanadium oxides |
hydration of SO3 gives H2SO4 |
| Ammonia synthesis (Haber–Bosch process) |
N2 + H2, NH3 |
iron oxides on alumina(Al2O3) |
consumes 1% of world's industrial energy budget |
| Nitric acid synthesis (Ostwald process) |
NH3 + O2, HNO3 |
unsupported Pt-Rh gauze |
direct routes from N2 are uneconomical |
| Hydrogen production by Steam reforming |
CH4 + H2O, H2 + CO2 |
Nickel or K2O |
Greener routes to H2 by water splitting actively sought |
Ethylene oxide synthesis |
C2H4 + O2, C2H4O |
silver on alumina, with many promotors |
poorly applicable to other alkenes |
| Hydrogen cyanide synthesis (Andrussov oxidation) |
NH3 + O2 + CH4, HCN |
Pt-Rh |
Related ammoxidation process converts hydrocarbons to nitriles |
| Olefin polymerization Ziegler–Natta polymerization |
propylene, polypropylene |
TiCl3 on MgCl2 |
many variations exist, including some homogeneous examples |
| Desulfurization of petroleum (hydrodesulfurization) |
H2 + R2S (idealized organosulfur impurity), RH + H2S |
Mo-Co on alumina |
produces low-sulfur hydrocarbons, sulfur recovered via the Claus process |
Other examples
- Reduction of nitriles for instance in a synthesis of phenethylamine with Raney nickel and ammonia:[11]
- The cracking, isomerisation and re-forming of hydrocarbons to form appropriate and useful blends of petrol.
- Catalytic converters are often used in automobiles. Three main reactions are catalysed by catalytic converters.
- The oxidation of carbon monoxide to carbon dioxide:
- 2CO(g) + O2(g) → 2CO2(g)
- The reduction of nitrogen monoxide back to nitrogen:
- 2NO(g) + 2CO(g) → N2(g) + 2CO2(g)
- The oxidation of hydrocarbons to water and carbon dioxide:
- 2 C6H6 + 15 O2 → 12 CO2 + 6 H2O
- This process can occur with any of the hydrocarbons, but most commonly is performed with petrol or diesel.
- Asymmetric heterogeneous catalysis affords enantiomerically enriched compounds using chiral heterogeneous catalysts.[12]
- The vast majority of heterogeneous catalysts are based on metals or metal oxides, however, some chemical reactions can be catalyzed by carbon-based materials, e.g., oxidative dehydrogenations[13] or selective oxidations.[14]
Ethylbenzene + 1/2 O2 → Styrene + H2O
Acrolein + 1/2 O2 → Acrylic acid
See also
- Homogeneous catalysis
- Temperature-programmed reduction
- Thermal desorption spectroscopy
- nanomaterial-based catalysts
- Platinum nanoparticles
- Heterogeneous gold catalysis
References
| Wikimedia Commons has media related to Heterogeneous Catalysis. |
^ Gadi Rothenberg, Catalysis: Concepts and green applications, Wiley-VCH: Weinheim, .mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
ISBN 978-3-527-31824-7
^ Swathi, R.S. and Sebastian, K.L. Molecular mechanism of heterogeneous catalysis. Resonance Vol. 13 Issue 6 (2008) p. 548-560.
^ Fritz Haber - Biographical
^ Carl Bosch - Biographical
^ Irving Langmuir - Biographical
^ Gerhard Ertl - Biographical
^ ab R. I. Masel, “Principles of Adsorption and Reaction on Solid Surfaces”, Wiley Series in Chemical Engineering, Wiley-Interscience, New York, USA, 1996,
ISBN 978-0-471-30392-3
^ Forzatti, P.; Lietti, L. (1999). "Catalyst Deactivation". Catalysis Today. 52: 165–181. doi:10.1016/S0920-5861(99)00074-7.CS1 maint: Uses authors parameter (link)
^ Bartholomew, Calvin H (2001). "Mechanisms of Catalyst Deactivation". Applied Catalysis A: General. 212 (1–2): 17–60. doi:10.1016/S0926-860X(00)00843-7.
^ Zhen Ma, Francisco Zaera "Heterogeneous Catalysis by Metals" in Encyclopedia of Inorganic Chemistry, 2006, John Wiley. doi:10.1002/0470862106.ia084
^ Organic Syntheses, Coll. Vol. 3, p.720 (1955); Vol. 23, p.71 (1943). https://web.archive.org/web/20120315000000*/http://orgsynth.org/orgsyn/pdfs/CV4P0603.pdf
^ Heitbaum, Glorius, Escher, Asymmetric heterogeneous catalysis, Angew. Chem. Int. Ed. 2006, 45, 4732.
^ Zhang, J.; Liu, X.; Blume, R.; Zhang, A.; Schlögl, R.; Su, D. S. (2008). "Surface-Modified Carbon Nanotubes Catalyze Oxidative Dehydrogenation of n-Butane". Science. 322 (5898): 73–77. Bibcode:2008Sci...322...73Z. doi:10.1126/science.1161916. PMID 18832641.
^ Frank, B.; Blume, R.; Rinaldi, A.; Trunschke, A.; Schlögl, R. (2011). "Oxygen Insertion Catalysis by sp2 Carbon". Angew. Chem. Int. Ed. 50 (43): 10226–10230. doi:10.1002/anie.201103340.


Comments
Post a Comment