Orellanine
 | |
| Names | |
|---|---|
IUPAC name 3,3′,4,4′-Tetrahydroxy-2,2′-bipyridine-N,N′-dioxide | |
| Other names Orellanin, 2,2-Bipyridine-3,3-4,4-tetrol-1,1-dioxide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChemSpider |
|
ECHA InfoCard | 100.232.424 |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C10H8N2O6 |
Molar mass | 7002252182000000000♠252.182 g·mol−1 |
| Hazards | |
| Main hazards | Highly toxic with delayed onset of toxicity |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Orellanine or orellanin is a mycotoxin found in a group of mushrooms known as the Orellani of the Cortinariaceae family.[1] Structurally, it is a bipyridine N-oxide compound somewhat related to paraquat
Contents
1 History
2 Synthesis
3 Toxicity
4 Treatment
5 See also
6 References
7 External links
History
Orellanine first came to people's attention in 1957 when there was a mass poisoning of 135 people in Bydgoszcz, Poland, which resulted in 19 deaths.[2][3][4] Orellanine comes from a class of mushrooms that fall under the genus Cortinarius. Although not all the species in this genus are poisonous/contain orellanine, it was found that Cortinarius orellanus, rubellus, henrici, rainerensis and bruneofulvus contain orellaine. Poisonings pertaining to these mushrooms were predominately in Europe where foraging was a major source of nourishment, although there are cases of orellanine poisoning in North America as well. Orellanine has been found to cause acute renal failure and there are many cases where people have taken this mushroom mistaking it for causing hallucinogenic effects.[5]
It wasn't until 1962 until the first isolation of orellanine was done. The first methanolic extraction and isolation of orellanine was done by Stanisław Grzymala and isolated from the mushroom Cortinarius orellanus.[4][6] Along with the isolation of orellanine, Grzymala was also able to demonstrate the nephrotoxicity of Cortinarius orellanus and determine various physical and chemical properties of orellanine. He found that the toxicity of the mushrooms was linked to delayed and acute renal failure, as well as when the isolated white crystalline substance was heated above 150 ̊C it began to slowly decompose.[3][4] After this first isolation of orellanine, the structure of orellanine was first discovered by Antkowiak and Gessner in 1979.[3][6] Orellanine’s structure was found to be 3,3',4,4'-tetrahydroxy- 2,2'-bipyridine-l,l'-dioxide. Antkowiak and Gessner were also able to determine that orellanine was the mono-N-oxide of orelline, which was the decomposition product of orellanine. It was also found that orelline was non-toxic. The first successful synthesis of orellanine was done in 1985. Tiecco, M. et. Al. completed a total synthesis of orellanine using commercially available 3-hydroxypyridine.[3] After the first successful synthesis, the structure was confirmed in 1987 by Cohen-Addad et al. in 1987 by X-ray crystallography.[6]
Synthesis
The chemical constitution of orellanine remained unknown until the Polish chemists Antkowiak and Gessner in the last half of the 1970s discovered that it was a bipyridine dioxide.[7][8] Orellanine undergoes tautomerization, and the more stable tautomer is the amine oxide form. An interesting feature of orellanine is its ability to bind aluminium ions to form chelation complexes.[9]

The first synthesis of orellanine was done in 1985 and Tiecco, M. et. Al. were able to synthesize orellaine from 3-hydroxy pyridine. This synthesis was completed in 8 steps with a 79-87% yield. When synthesized the two pyridyl rings are nearly perpendicular and the molecule is chiral.[4] When it is isolated from the mushroom, it is an optically inactive racemic mixture. Other synthetic strategies have also been attempted. For example, orellanine was also synthesized in 9 steps by Dehmlow and Schulz in 1985 using 3-aminopyridine and the desired product was synthesized with 30% yield.[2]
In the synthesis done by Tiecco, M. et al. can be seen in the scheme below. In the first step, 3-hydroxy pyridine was first treated with bromine in an alkaline solution to obtain 2. The product of that step was then subjected to O-alkylation using DMF as a solvent to obtain 3. 3 was the oxidized with m-chloroperbenzoic acid in chloroform to give 4. That product was then nitrated with nitric acid and sulphuric acid to obtain a mixture of 5 and 6. These two molecules were separated by combining the mixture of products with water. 5 is insoluble in water, whereas 6 is soluble in water. 6 was then subjected to sodium methoxide in methanol to obtain the other methoxy group seen in product 7 and 7 was deoxygenated using phosphorus tribromide to obtain 8. To obtain tetramethyl orelline, structure 9, triphenylphosphine, NiCl2·6H2O, and zinc powder were used to conduct the homocoupling of halopyridines through the use of nickel-phosphine complexes. Once 8 with the bipyridyl structure had been obtained, the synthesis of orellanine could be conducted. The bipyridyl product from number 8 is then dealkylated with hydrobromic acid to give orelline, which was found to be a yellow crystalline solid (10). Orelline is then oxidized with hydrogen peroxide using heat to obtain the desired product, orellanine (11).
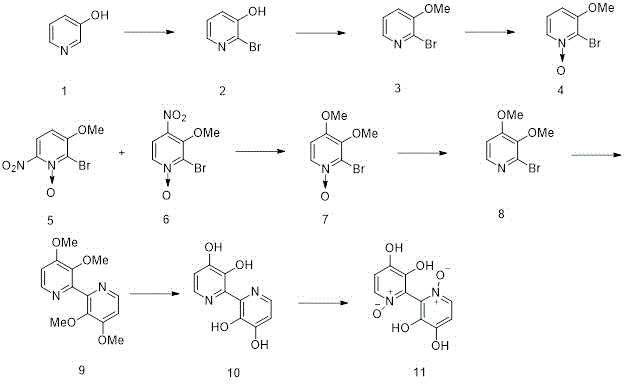
Another way to synthesize orellanine from 9 was to subject it to excess m-chloro perbenzoic acid in chloroform to obtain 12, tetramethyl orellanine. Tetramethyl orellaine was then demethylated using hydrobromic acid to yield orellanine.

Toxicity
Bipyridines with positively charged nitrogen atoms were already known to be poisonous before the structure of orellanine was elucidated. The herbicides paraquat and diquat are toxic not only to plants, but also to humans and livestock. Bipyridines with charged nitrogen atoms disrupt important redox reactions in organisms, 'stealing' one or two electrons and sometimes bypass the electrons into other, often undesirable, redox reactions. The terminal product can be peroxide or superoxide ions, the latter of which are harmful to the cells. It is likely that orellanine works in the same way, although the process that leads from disturbed redox reactions to serious clinical kidney damage has not been properly resolved.
In humans, a characteristic of poisoning by the nephrotoxin orellanine is the long latency; the first symptoms usually do not appear until 2–3 days after ingestion and can in some cases take as long as 3 weeks. The first symptoms of orellanine poisoning are similar to the common flu (nausea, vomiting, stomach pains, headaches, myalgia, etc.), these symptoms are followed by early stages of renal failure (immense thirst, frequent urination, pain on and around the kidneys) and eventually decreased or nonexistent urine output and other symptoms of renal failure occur. If left untreated death will follow.
The LD50 of orellanine in mice is 12 to 20 mg per kg body weight;[10][11] this is the dose which leads to death within two weeks. From cases of orellanine-related mushroom poisoning in humans it seems that the lethal dose for humans is considerably lower.
Treatment
Although there is no known antidote against orellanine poisoning, early hospitalization can sometimes prevent serious injury and usually prevent death. Research is ongoing. Some treatments make use of anti-oxidant therapy and corticosteroids to help victims recover from their renal failure.[12]
See also
- Lethal webcaps
- Cortinarius
- Paraquat
References
^ Oubrahim H.; Richard J.-M.; Cantin-Esnault D.; Seigle-Murandi F.; Trecourt F (1997). "Novel methods for identification and quantification of the mushroom nephrotoxin orellanine". Journal of Chromatography. 758 (1): 145–157. doi:10.1016/S0021-9673(96)00695-4. PMID 9181972..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ ab Dehmlow, Eckehard V.; Schulz, Hans-Joachim (1985). "Synthesis of orellanine the lethal poison of a toadstool - ScienceDirect". Tetrahedron Letters. 26 (40): 4903–4906. doi:10.1016/S0040-4039(00)94981-5.
^ abcd Tiecco, Marcello; Tingoli, Macro; Testaferri, Lorenzo; Chianelli, Donatella; Wenkert, Ernest (1985). "Total synthesis of orellanine : The lethal toxin of Cortinarius orellanus fries mushroom - ScienceDirect" (PDF). Tetrahedron. 42 (5): 1475–1485. doi:10.1016/S0040-4020(01)87367-1.
^ abcd Dinis-Oliveira, Ricardo Jorge; Soares, Mariana; Rocha-Pereira, Carolina; Carvalho, Félix (2015). "Human and experimental toxicology of orellanine". Human & Experimental Toxicology. 35 (9): 1016–1029. doi:10.1177/0960327115613845. PMID 26553321.
^ Deutsch, Christopher J.; Swallow, Daniel (24 September 2012). "Deliberate ingestion of "magic" mushrooms may also cause renal failure". BMJ. 345: e6388. doi:10.1136/bmj.e6388. ISSN 1756-1833. PMID 23008206.
^ abc Richard, Jean-Michel; Louis, Josette; Cantin, Danielle (1 March 1988). "Nephrotoxicity of orellanine, a toxin from the mushroom Cortinarius orellanus". Archives of Toxicology. 62 (2–3): 242–245. doi:10.1007/BF00570151. ISSN 0340-5761.
^ Antkowiak W.Z., Gessner W. P. (1975). "Isolation and characteristics of toxic components of Cortinarius orellanus, Fries". Bulletin de l'Academie Polonaise des Sciences, Serie des Sciences Chemiques. 23: 729–733.
^ Antkowiak Wieław Z., Gessner Wiesł P. (1979). "The structures of orellanine and orelline". Tetrahedron Letters. 20 (21): 1931–1934. doi:10.1016/s0040-4039(01)86882-9.
^ Høiland K (1994). "Suppression of the toxic effect of soluble aluminium on fungi by dermocybin-1-ß-D-glucopyranoside and orellanine from Cortinarius sanguineus and C. orellanoides". Nord. J. Bot. 14 (2): 221–228. doi:10.1111/j.1756-1051.1994.tb00591.x.
^ Prast H; Werner ER; Pfaller W; Moser M. (1988). "Toxic properties of the mushroom Cortinarius orellanus. I. Chemical characterization of the main toxin of Cortinarius orellanus (Fries) and Cortinarius speciosissimus (Kuhn & Romagn) and acute toxicity in mice". Archives of Toxicology. 62 (1): 81–8. doi:10.1007/bf00316263. PMID 3190463.
^ Holmdahl, J (2001). Mushroom poisoning: Cortinarius speciosissimus nephrotoxicity (PDF). Göteborg University. Archived from the original (PDF) on February 4, 2012.
^ Rachael G. Kilner; et al. (1999). "Acute renal failure from intoxication by Cortinarius orellanus: recovery using anti-oxidant therapy and steroids". Nephrology Dialysis Transplantation. 14 (11): 2779–2780. doi:10.1093/ndt/14.11.2779-a.
External links
Cortinarius rubellus Pacific Northwest Fungi, Featured Fungus Number 4

Comments
Post a Comment