Incandescence

Hot metal work glows with visible light. This thermal radiation also extends into the infrared, invisible to the human eye and the camera the image was taken with, but an infrared camera could show it (See Thermography).

The incandescent metal embers of the spark used to light this Bunsen burner emit light ranging in color from white to orange to red or to blue. This change correlates with their temperature as they cool in the air. The flame itself is not incandescent, as its blue color comes from the quantized transitions that result from the oxidation of CH radicals.
Incandescence is the emission of electromagnetic radiation (including visible light) from a hot body as a result of its temperature.[1] The term derives from the Latin verb incandescere, to glow white.[2]
Incandescence is a special case of thermal radiation. Incandescence usually refers specifically to visible light, while thermal radiation refers also to infrared or any other electromagnetic radiation.
For information on the intensity and spectrum (color) of incandescence, see thermal radiation.
Contents
1 Observation and use
2 Figurative use
3 See also
4 References
5 External links
Observation and use
In practice, virtually all solid or liquid substances start to glow around 798 K (525 °C) (977 ˚F), with a mildly dull red color, whether or not a chemical reaction takes place that produces light as a result of an exothermic process. This limit is called the Draper point. The incandescence does not vanish below that temperature, but it is too weak in the visible spectrum to be perceivable.
At higher temperatures, the substance becomes brighter and its color changes from red towards white and finally blue.
Incandescence is exploited in incandescent light bulbs, in which a filament is heated to a temperature at which a fraction of the radiation falls in the visible spectrum. The majority of the radiation however, is emitted in the infrared part of the spectrum, rendering incandescent lights relatively inefficient as a light source.[3] If the filament could be made hotter, efficiency would increase; however, there are currently no materials able to withstand such temperatures which would be appropriate for use in lamps.
More efficient light sources, such as fluorescent lamps and LEDs, do not function by incandescence.[4]
Sunlight is the incandescence of the "white hot" surface of the sun.
Figurative use
The word incandescent is also used figuratively to describe a person who is so angry that they are imagined to glow or burn red hot or white hot.[5]
See also
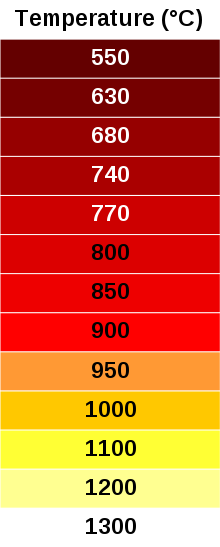
The visible color of an object heated to incandescence (from 550°C to 1300°C (1022°F to 2372°F))
- Red heat
- List of light sources
References
^ Dionysius Lardner (1833). Treatise on Heat. Longman, Rees, Orme, Brown, Green & Longman. Archived from the original on 2017-12-21.The state in which a heated body, naturally incapable of emitting light, becomes luminous, is called a state of incandescence.
.mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ John E. Bowman (1856). An Introduction to Practical Chemistry, Including Analysis (Second American ed.). Philadelphia: Blanchard and Lea. Archived from the original on 2017-12-21.
^ William Elgin Wickenden (1910). Illumination and Photometry. McGraw-Hill. Archived from the original on 2017-12-21.
^ Koones, Sheri (2012-10-01). Prefabulous + Almost Off the Grid: Your Path to Building an Energy-Independent Home. Abrams. ISBN 9781613123966.
^ Example 1:'...the stadium positively crackled with the incandescent anger of anguished supporters.' Mark Wilson, 'Rangers 1 Unirea 4', Daily Mail, 21 October 2009 "Archived copy". Archived from the original on 2012-08-02. Retrieved 2009-11-01.CS1 maint: Archived copy as title (link). Example 2: '...there's something very funny about incandescent anger.' Mark Fisher, 'Jerry has a cross to bear', The Scotsman, 5 March 2006 [1].

Comments
Post a Comment